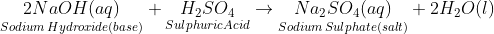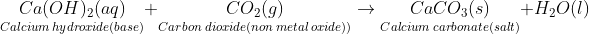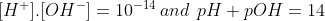Bases
- Bases are those chemical substances that have a bitter taste.
- All the bases change the colour of Red litmus to blue.
- A base is a chemical substance that can neutralise an acid.
- A common property of all the bases(or alkalis) is that they all produce hydroxide ions(OH ions) when dissolved in water.
Most of the bases do not dissolve in water but some bases dissolve in water thus, a base that is soluble in water is called an alkali.
Strong Bases:
A base that completely ionises in water and produces a large number of hydroxide ions (OH ions) is called a strong base(or a strong alkali) Ex:- KOH, NaOH
Weak Bases:A base that is partially ionised in water and thus produces a small number of hydroxide ions(OH ions) is called a weak base(or weak alkali)

Properties of Bases
- Bases have a bitter taste
- Bases feel soapy to touch
- Bases turn red litmus to Blue
- Bases conduct electricity in solution(they are electrolytes)
- Bases react with some metals to form hydrogen gas
- Bases react with acids to form salt and water
- Bases react with metal oxides to form salt and water
Uses Of Bases
- Sodium hydroxide is used in the manufacture of soap, paper and a synthetic fibre called 'rayon'.
- Calcium hydroxide(called slaked lime) is used in the manufacture of bleaching powder.
- Magnesium hydroxide is used as an antacid to neutralise excess acid in the stomach and cure indigestion.
- Sodium carbonate is used as washing soda and for softening hard water.
- Sodium hydrogen carbonate is used as baking soda in cooking food for making baking powders, as an antacid to cure indigestion and in soda-acid fire extinguishers.
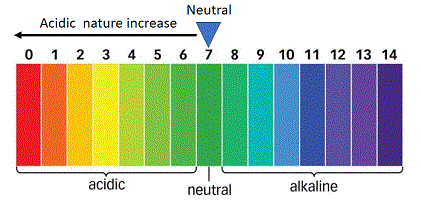
pH Scale
Note:-The letter pH stands for Potez of hydrogen means the power of hydrogen in the Denish language, because first time Denish scientist Sorensen explain this. According to the rules of the pH scale
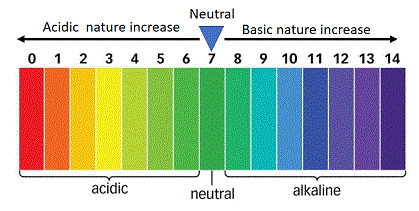
- Neutral substances have a pH of exactly 7.
- Acids(acidic solutions) have a pH of less than 7
- Bases(or basic solutions) have a pH of more than 7.
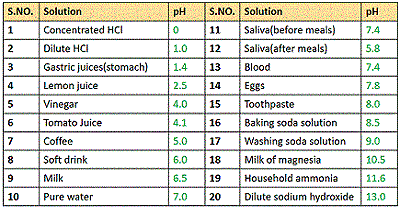
pH values of some common substances
Universal Indicator
The common indicators(like litmus) can tell us whether the given substance is an acid or base. They can not tell us whether the given substance is a strong acid, a weak acid,.a strong base or a weak base.
A common method of measuring the pH of a solution in the school laboratory is to use a universal indicator. A universal indicator is a mixture of many different indicators(or dyes) which gives different colours at different pH values of the entire PH scale.
The colours produced by the universal indicator at various pH values are given below:
The colour produced by universal indicator paper at various pH values are shown in the chart given
Importance of pH in everyday life
1:-pH in our digestive system
Our stomach produces HCl.This diluted HCl acid help in digesting our food. The excess acid in the stomach causes indigestion. To cure indigestion we take a base called 'Antacids'.Antacids are a group of mild bases which have no toxic effects on the body.
Antacids are basic so react with the acid in the stomach and neutralise it. The two common antacids used for curing indigestion due to acidity are: Magnesium hydroxide(milk of Magnesia) and Sodium hydrogen carbonate(Baking Soda)
2: pH cause of Tooth Decay
Tooth decay starts when the pH of acid formed in the mouth falls below 5.5.The acid becomes strong enough to attack the enamel(made of Calcium phosphate hardest material in our body) of our teeth and corrode it. This sets in our tooth decay.
- The best way to prevent tooth decay is to clean the mouth after eating food.
- Toothpaste is slightly basic. It helps prevent tooth decay by neutralising the excess acid in our mouth.
3:Plants and animals are sensitive to pH changes
- Most plants grow best when the pH of the soil is close to 7.
- If the soil is too acidic then it is treated with materials like quicklime(CaO) or salked lime(calcium hydroxide) or chalk(calcium carbonate)
- The pH plays an important role in the survival of animals, including human beings. Our bodies work well within a narrow pH range of 7.0 to 7.8.
4:Self defence by animals and plants by chemical warfare
- Many animals and plants protect themselves by injecting painful and irritating acids and bases into their skin.
- When a honey bee stings a person, it injects an acidic liquid into the skin which causes pain and irritation. Rubbing baking soda(base) at the sting area of the sting gives relief.