Nuclear Energy:
A physical reaction that involves changes in the nucleus of an atom is called a nuclear reaction. The energy released during a nuclear reaction is called nuclear energy. Nuclear energy can be obtained by two types of nuclear reactions.- Nuclear fission
- Nuclear fusion
[1] Nuclear Fission:
The word fission means to split up into two or more parts. The process in which the heavy nucleus of a radioactive atom(such as uranium, plutonium, or thorium) splits up into smaller nuclei when bombarded with low energy neutrons, called nuclear fission.
Fission was discovered in 1934 when Enrico Fermi of Italy, irradiated uranium with neutrons and believed he had produced the first transuranic element. In 1938 Otto Hahn and Fritz Strassmann of Germany split the uranium atom by bombarding it with neutrons and showed that the elements barium and krypton were formed.
When uranium-235 atoms are bombarded with a slow-moving neutron, the heavy uranium nucleus breaks up to produce two medium-weight atoms,barium-139 and krypton-94 with the emission of 3 neutrons. A tremendous amount of energy is produced during the fission of uranium. This fission reaction can be represented in the form of a nuclear equation as:
Fission was discovered in 1934 when Enrico Fermi of Italy, irradiated uranium with neutrons and believed he had produced the first transuranic element. In 1938 Otto Hahn and Fritz Strassmann of Germany split the uranium atom by bombarding it with neutrons and showed that the elements barium and krypton were formed.
When uranium-235 atoms are bombarded with a slow-moving neutron, the heavy uranium nucleus breaks up to produce two medium-weight atoms,barium-139 and krypton-94 with the emission of 3 neutrons. A tremendous amount of energy is produced during the fission of uranium. This fission reaction can be represented in the form of a nuclear equation as:
 |
| Nuclear Fission |
Reaction formula for fission is here:-
In the fission of uranium, some mass of uranium disappears, and a tremendous amount of energy is produced. 1atom of uranium-235 produced 10 million times more energy than the energy produced by the burning of 1 atom of carbon from coal.
Use of Fission: -
Use of Fission: -
- Nuclear Bomb or Atom Bomb ( Use Uncontrollable Chain Reaction)
- Nuclear Power Plant (Use Controllable chain Reaction)
The U.S. developed two types of atomic bombs during the Second World War. The first, Little Boy, was a gun-type weapon with a uranium core. Little Boy was dropped on Hiroshima. The second weapon, dropped on Nagasaki, was called Fat Man and was an implosion-type device with a plutonium core.
 |
| Nuclear bomb test explosion |
The Difference Between the Little Boy and Fat Man:
Little Boy and Fat Man utilized different elements and completely separate methods of construction in order to function as nuclear weapons. Little Boy detonated due to a fission chain reaction involving the isotope U-235 of uranium, while Fat Man used plutonium’s Pu-239 form.
 |
| Construction difference between little boy and fat man |
Little Boy:
Little Boy was powered by the uranium isotope U-235 follow the gun-type design. Most uranium found naturally in the world exists as uranium-238, leaving only 0.7% of naturally existing uranium as the U-235 isotope. When a neutron bombards U-238, the isotope often captures the neutron to become U-239, failing to fission, and thus failing to instigate a chain reaction that would detonate a bomb.
Powered by plutonium, Fat Man could not use the same gun-type design that allowed Little Boy to explode effectively Fat Man Use
implosion-type design - the form of plutonium collected from the nuclear reactors.
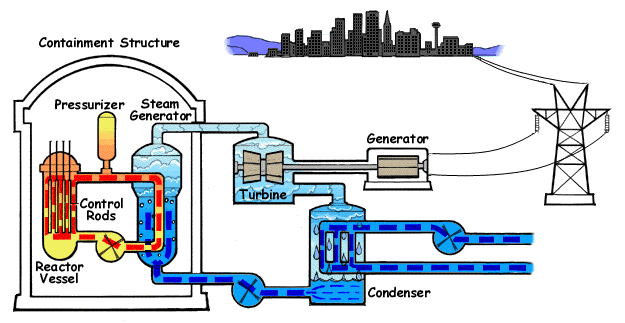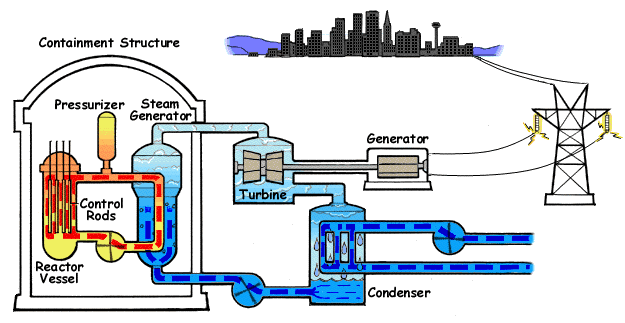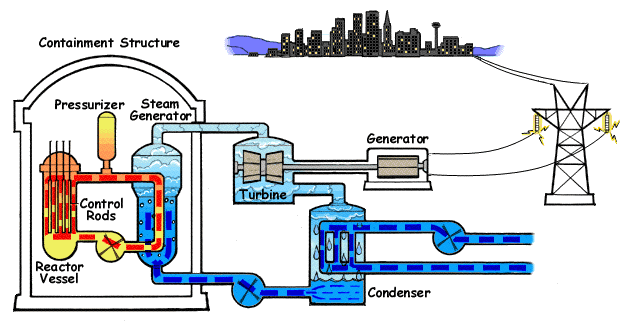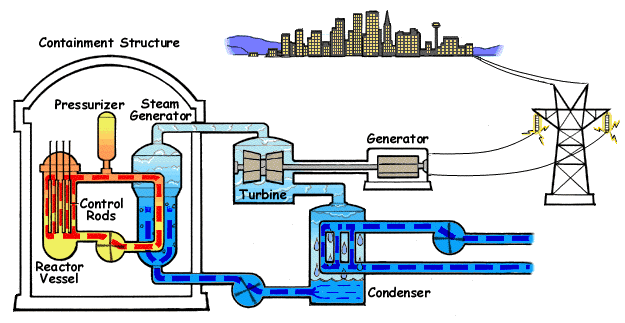
A power plant in which the heat required to make steam and turn turbine(to drive generators for making electricity) is obtained by nuclear reactions called power plat. Nuclear power plants use a nuclear reaction to generate electricity. Most of the nuclear power plant uses uranium 235 as fuel to produce heat. But uranium 235 fuel is not burnt like coal, oil or gas to obtain energy. Its energy is related to the nuclear fission process.
Component of Nuclear Power Plant:
Nuclear Power Plants in India:
Einstein Mass-energy Relation:
 |
| Nuclear Power Plant |
Component of Nuclear Power Plant:
- Reactor Core: The reactor core is made -up of stainless steel or zirconium.
- Moderator: It decreases the speed of neutron. (1) Heavy water D2O (2) Graphite (3) Beryllium, order of moderator is heavy water>Berllium>Graphite
- Reflector: Prevents escape of neutrons from the reactor core and made-up of high-grade graphite.
- Control Rod: During an earthquake, it trips the generating station(Boron Rod).
- Coolant: Heavy Water, Na, Li are used as coolant material.
- Shielding: Shielding eliminates the effect of radiation.
- Kundankulam Nuclear Power Plant, Tamil Nadu
- Tarapur Nuclear Power Plant, Maharashtra
- Rajasthan Atomic Power Plant, Rajasthan
- Kaiga Atomic Power Plant, Karnataka
- Kalpakkam Nuclear Power Plant, Tamil Nadu
- Narora Nuclear Power plant Uttar Pardesh
- Kakarapar Atomic Power Plant, Gujrat
- At present only about 3 to 4% of the total electric power produced in India is obtained from nuclear power plants. A country like France, Germany, etc, more than 30% of their total electrical power comes from nuclear power plants.
Einstein Mass-energy Relation:
Einstein said that mass and energy are equivalent and are related by the equation:
E=mc2
The equation made famous by Albert Einstein. Explains how a tiny amount of matter contains a tremendous amount of energy.
Thus 1kg mass-produced a huge amount of energy of 9×1016j
Energy Units For expressing nuclear energy:
We know that that common unit of energy is the joule(j). But the energy-related in a nuclear reaction is expressed in the units of 'electron Volt(eV)' or Million electron Volt(MeV). 1 electron volt is the amount of energy acquired by an electron when accelerated through a potential difference of 1 volt.
1 electron Volt=1.602×10−19j
or,1eV=1.602×10−19j
or,1eV=1.602×10−19j
1Mev=1million×1ev=106×1.602×10−19
=1.602×10−13j
=1.602×10−13j
The Value of Atomic Mass Unit in Terms of Energy:
1atomic.mass.unit(u)=1.492×10−10j
1 u = 931 Mev
Thus 1 atomic mass unit(u) is equivalent to 931 Mev of energy.
[2] Nuclear Fusion(Uncontrollable Chain Reaction):
The word 'fusion' means 'to join' or' to combine'. The process in which two nuclei of light elements(like that of hydrogen) combine to form a heavy nucleus(like that of helium) is called nuclear fusion. A tremendous amount of energy is produced during the fusion process. We know that the nuclei of atoms are positively charged. So, when two nuclei are brought together, they repel each other due to their similar charges. Due to this a lot of initial heat energy and high pressure are required to force the lighter nuclei to fuse together to form a bigger nucleus. So, the conditions needed for carrying out the nuclear fusion processes are millions of degrees of temperature and million of Pascal's of pressure. In other words, nuclear fusion is carried out by heating the lighter atoms to extremely high temperatures under extremely high pressure. There is some loss of mass during the fusion process which appears as a tremendous amount of energy.
Here is the example of a nuclear fusion reaction.
When deuterium atoms (heavy hydrogen atoms of mass number 2) and Tritium atoms (heavy hydrogen atoms of mass number 3) are heated to an extremely high temperature under extremely high pressure, then they combine together to form a heavy nucleus of helium and a neutron is emitted. A tremendous amount of energy is liberated in this fusion reaction. This fusion reaction can be written as:

A fusion process is just the opposite of the fission process. The energy produced in a nuclear fusion reaction is, however, much more than that produced in a nuclear fission reaction.
The energy produced during nuclear fusion has not been controlled so far. So, nuclear fusion energy could not be used for generating electricity so far
When deuterium atoms (heavy hydrogen atoms of mass number 2) and Tritium atoms (heavy hydrogen atoms of mass number 3) are heated to an extremely high temperature under extremely high pressure, then they combine together to form a heavy nucleus of helium and a neutron is emitted. A tremendous amount of energy is liberated in this fusion reaction. This fusion reaction can be written as:
A fusion process is just the opposite of the fission process. The energy produced in a nuclear fusion reaction is, however, much more than that produced in a nuclear fission reaction.
The energy produced during nuclear fusion has not been controlled so far. So, nuclear fusion energy could not be used for generating electricity so far
 |
| Fusion reaction |
Application of Nuclear Fusion:
- Hydrogen Bomb(Uncontrollable chain reaction)
- The Source of Sun's Energy(Uncontrollable chain reaction)
[1] Hydrogen Bomb(Uncontrollable chain reaction):
Thermonuclear reactions(fusion reactions which occur at very high temperature) are used for producing a weapon of mass destruction called a hydrogen bomb. The hydrogen bomb consists of heavy isotopes of hydrogen called deuterium and tritium along with an element lithium-6. The detonation(or explosion) of the hydrogen bomb is done by using an atom bomb(based on the fission of uranium-235 or plutonium-239). When the atom bomb is exploded, then its fission reaction produced a lot of heat. This heat raises the temperature of deuterium and tritium to 10^{7}℃ in a few microseconds. At this temperature fusion reaction of deuterium and tritium take place producing a tremendous amount of energy. The function of lithium-6 used in the hydrogen bomb is to produce more tritium needed for fusion.
 |
| Hydrogen bomb |
Please note that a hydrogen bomb is much more powerful than an atom bomb .A hydrogen bomb is actually an uncontrolled nuclear fusion process. The source of energy of a hydrogen bomb is the same as that of the Sun's energy.
[2] The Source of Sun's Energy:
The sun is a huge mass of hydrogen gas and the temperature in it is extremely high. The sun may be considered a big thermonuclear furnace where hydrogen atoms are continuously being fused into helium atoms. Mass is being lost during these fusion reactions and energy is being produced. Thus, the sun which gives us heat and light derives its energy from the fusion of hydrogen nuclei into helium nuclei, which is going on inside it, all the time.
The main nuclear fusion reaction taking place in the sun which releases a tremendous amount of energy is the fusion of 4 hydrogen atoms nuclei to form a bigger nucleus of helium atoms. that is...

Nuclear fusion reactions of hydrogen are the source of the sun's energy. Please note that just like the sun, other stars also obtain their energy from the nuclear fusion reaction of hydrogen.
Advantage of Nuclear Energy:
The main nuclear fusion reaction taking place in the sun which releases a tremendous amount of energy is the fusion of 4 hydrogen atoms nuclei to form a bigger nucleus of helium atoms. that is...
Nuclear fusion reactions of hydrogen are the source of the sun's energy. Please note that just like the sun, other stars also obtain their energy from the nuclear fusion reaction of hydrogen.
Advantage of Nuclear Energy:
- It produces a large amount of useful energy from a very small amount of nuclear fuel (like uranium-235)
- Once the nuclear fuel (like uranium-235) is loaded into the reactor, the nuclear power plant can go on producing electricity for two or three years at a stretch. There is no need for putting in nuclear fuel again and again.
- It does not produce gases like carbon dioxide contributes to the greenhouse effect or sulfur dioxide which causes acid rain.
- The waste products of nuclear fission reactions(produced at nuclear power plants) are radioactive which keep on emitting harmful nuclear radiations for thousands of year. So, it is very difficult to store or disposes of nuclear waste safely. Improper nuclear waste storage or disposal can pollute the environment.
- There is the risk of accidents in nuclear reactors(especially the old nuclear reactors). Such accidents lead to the leakage of radioactive materials which can cause serious damage to the plants, animals, and the environment.
- The high cost of installation of nuclear power plants and the limited availability of uranium fuel make the large scale use of nuclear energy prohibitive.




